Validated GMO inactivation
In order to more formally validate the usual GMO inactivation done with bleach for disposal of plates and cultures, we are adding this page. This validation was performed with two brands of commercial bleach and is applicable to both GMO bacteria and nematode worms transformed with antibioticR plasmids. It is directly specific for this SOP of the lab collection.
As always, precautions in using bleach and appropriate personal protective equipment (lab coat, gloves, and eye protection) are an essential part of this inactivation method.
The big white tray - labelled 'clean up' - was used, as usual for all manipulations.
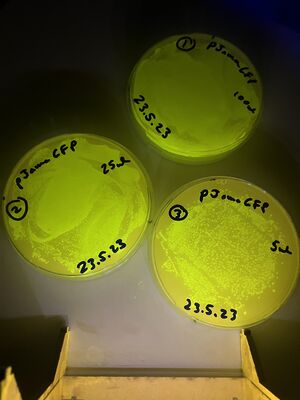
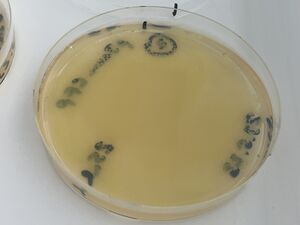
The validation for bacterial plates was done upon bacteria transformed with a pJAMA-GFP plasmid, which make bright green colonies...
A fresh 2ml culture was used to make three plates of cultures on growth media carrying the selective antibiotic, ampicillin (LB-amp100 plates). A glass spreader was used - from a bent pasteur pipette, to distribute the following liquids.
1, with 100ul of culture (full lawn)
2, with 25ul of culture (less bugs)
3, with 5ul of culture (still too many to count)
These are shown below:
Then, the plates were treated with bleach in the big white tray.
Three conditions of inactivation for this validation experiment were run: full strength bleach, for plate 1, 1/10 bleach in water for plate 2, and water only for plate 3, with the least colonies.
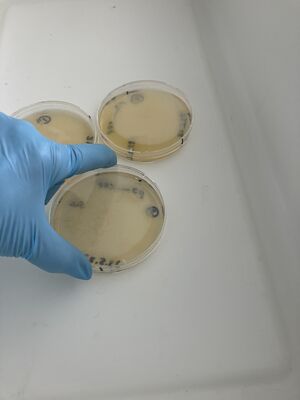
5ml of each solution was added to the appropriate plate, incubated for 5min and then swirled, as shown below, and incubated 5 more minutes.
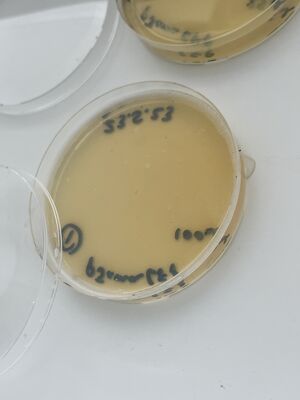
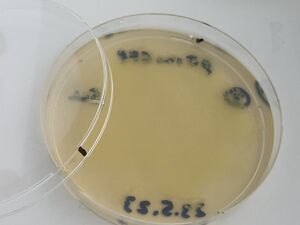
Then, after waiting 5 more minutes, a couple of mls of the liquid from each plate was recuperated into small conical tubes (15ml) with sterile serological pipets and the old Drummond pipet-aid. On plate 1, all colonies appeared fully dissolved by the undiluted bleach, but plate 2 with 1/10 bleach, and plate 3, with only water, still had intact colonies, as shown.
From the recuperated liquids, three more fresh plates were made by inoculating 50ul of each liquid taken after the treatment, as shown.
After all that, a repeat of the inactivation of all three initial plates, as per the SOP, with 100% javel put also on plate 2 and 3 was performed (esp as it was clear even the 1/10 solution is not dissolving the colonies, like the full strength one. The 'clean up' tray was carefully washed in back big sink, as usual (around in the intermediate Polymeka space).
The 9 new plates were incubated overnight at 37oC and checked for growth.