Difference between revisions of "Grow&Mix Bioink"
(→Who) |
(→How) |
||
| Line 29: | Line 29: | ||
Pr(ink)t plastic, it's fantastic! | Pr(ink)t plastic, it's fantastic! | ||
| − | + | STRAINS: | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
J. Lividum | J. Lividum | ||
M. Luteus | M. Luteus | ||
| Line 91: | Line 35: | ||
M. Aurantica | M. Aurantica | ||
| − | + | CULTURES: | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
LB medium (liquid, no agar) | LB medium (liquid, no agar) | ||
See LB protocol [http://wiki.hackuarium.ch/w/BioP_Cultures here] | See LB protocol [http://wiki.hackuarium.ch/w/BioP_Cultures here] | ||
| − | + | STERILISATION: | |
| + | Autoclave: | ||
0.-Plug | 0.-Plug | ||
1.-fill the bottom with water till reach the 1st level/grid on the bottom. | 1.-fill the bottom with water till reach the 1st level/grid on the bottom. | ||
| Line 114: | Line 50: | ||
7.-open (not open till it has reached the room t°) | 7.-open (not open till it has reached the room t°) | ||
8.-Unplug | 8.-Unplug | ||
| − | + | ||
| − | + | ||
| − | |||
EXTRACTIONS: | EXTRACTIONS: | ||
First take some culture from the original bottle, "source". Prepare the table: | First take some culture from the original bottle, "source". Prepare the table: | ||
| Line 124: | Line 59: | ||
empty the source bottle on the new bottle | empty the source bottle on the new bottle | ||
heat it up a bit with fire the mouth of the bottle and the aluminium foil before putting in again the aluminium foil. | heat it up a bit with fire the mouth of the bottle and the aluminium foil before putting in again the aluminium foil. | ||
| − | |||
Then we continue the process with this tools: | Then we continue the process with this tools: | ||
| − | |||
1.- Pipette, always use the plastic cone(aeroject) on them: We extract some an put them in tubes (then close them). | 1.- Pipette, always use the plastic cone(aeroject) on them: We extract some an put them in tubes (then close them). | ||
| − | |||
2.- Then we shake the tubes in the automatic vortex at 10 x 1000 and 10 minutes | 2.- Then we shake the tubes in the automatic vortex at 10 x 1000 and 10 minutes | ||
| − | |||
3.- Empty the lb medium that should remain on the top, we mix the rest (the pigment), with alcohol, we put the tubes in the machine of ultrasounds, in order to break the molecules to free the pigment. (ultra strong detergent would be helpful) the tube content should seem creamy and colorful. | 3.- Empty the lb medium that should remain on the top, we mix the rest (the pigment), with alcohol, we put the tubes in the machine of ultrasounds, in order to break the molecules to free the pigment. (ultra strong detergent would be helpful) the tube content should seem creamy and colorful. | ||
| − | |||
4.- and then we put them again into the vortex in order to compact the pigment and keep it as a first sample to work with, THE FIRST YELLOW INK!!! | 4.- and then we put them again into the vortex in order to compact the pigment and keep it as a first sample to work with, THE FIRST YELLOW INK!!! | ||
| + | **** Notes on J. Lividum*********** | ||
| + | X.Perret, Senior Lecturer Microbiology Unit Laboratory of Microbial Genetics Sciences III – University of Geneva has accepted to collaborate in the project. We are working in the lab at UM under his supervision and the assistance of his students and Phd students. First Extraction following the article Pantanella-F_2007: [http://www.ncbi.nlm.nih.gov/pubmed/17381742] | ||
| − | + | Culture on TY and TY + Glycerol | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
SOME CONCLUSIONS: | SOME CONCLUSIONS: | ||
| Line 217: | Line 76: | ||
LB + 4gr. plus of "food" (yeast and peptone) neither. MLuteus grow better on LB agar and liquid medium at 37°C (maybe not well calibrated machine...) | LB + 4gr. plus of "food" (yeast and peptone) neither. MLuteus grow better on LB agar and liquid medium at 37°C (maybe not well calibrated machine...) | ||
| − | + | Extraction: | |
| − | ---- | + | Violacein extraction |
| − | + | J. lividum was grown in 2 ml of LB or LB-U or LB-Y in 24-well plates. At regular time intervals, bacterial cells from a single well were harvested by centrifugation (16 000 g for 20 min), and treated for violacein extraction. Violacein extraction was carried out according to previously described methods (Blosser and Gray 2000; Matz et al. 2004). Briefly, bacterial cells were lysed with 10% sodium dodecyl sulfate. After 5 min at room temperature, water-saturated butanol was added (1 : 2 v/v) and vortexed. After centrifugation (16 000 g for 10 min) the upper phase, containing violacein, was collected. The extracted violacein was quantified using a spectrophotometer (OD585). The extinction coefficient of violacein (0Æ05601 ml lg)1 cm)1) was used for quantitative calculations (Mendes et al. 2001). | |
| + | ************************************ | ||
| − | + | EXTRUSIONS--> biocoloured bioplastic: | |
| − | + | Building the FilaBot DIY Kit, an extruder for plastics. 40 hours of intense work. Thank Michael for the moral support and electronic supervision. | |
| − | + | Put the natural white pellets on the machine and verted the bio+ink. | |
| − | |||
| − | |||
The problem seems to be solved almost, if the pigments are added BEFORE putting them into the extruder let them dry (because of the extraction they already have alcohol so they dry fast) for a around 30min at room T°. | The problem seems to be solved almost, if the pigments are added BEFORE putting them into the extruder let them dry (because of the extraction they already have alcohol so they dry fast) for a around 30min at room T°. | ||
| − | + | RESULT: | |
| − | |||
We could start printing with our Pr(ink)t as soon as I get to assembly and connect it! | We could start printing with our Pr(ink)t as soon as I get to assembly and connect it! | ||
| − | + | [https://vlorenzolana.myportfolio.com/prinkt-plastic-its-fantastic RESULT OFFICIAL PAGE PRINT PLASTIC IS FANTASTIC] | |
<br> | <br> | ||
Revision as of 11:30, 8 December 2016
What
What if we could produce colors and plastic from bacteria in our kitchens? What if we could create materials, colors and machines for building our objects? DIY is the idea of doing by yourself those activities reserved to the industrial production system that encourages alienation and non-participation. Imagine people producing machines ink and biomaterials out of microorganisms found in Nature and brought to their kitchens. Project developed at Hackuarium with the collaboration of Prof. Xavier Perret. Conceptualized during my first semester at HEAD Geneva, the result was exhibited at Fiera del Mobile di Milano in 2015.
Who
Vanessa Lorenzo
Hackuarium team that helped to push this project forward: Sachiko Kana, Michael Pereira, Octanis Team, Luc Henry, Yann Pierson, Gianpaolo Rando, Yann Heurtaux, Carmelo Bisognano (Univercité), Clément Drévo and Gavrilo Bozovic.
Xavier Perret UNIGE that provided proof of concept experiment with the Violacein at his lab.
Naiyi Wang that designed infographics
Alexandra Midal and Mathieu Bassée HEAD Genève that promoted the early project in 2015.
How
Pr(ink)t plastic, it's fantastic!
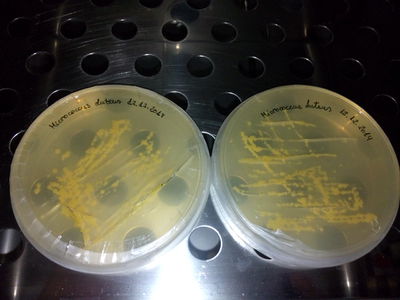
STRAINS: J. Lividum M. Luteus M. Roseus M. Aurantica
CULTURES: LB medium (liquid, no agar) See LB protocol here
STERILISATION: Autoclave: 0.-Plug 1.-fill the bottom with water till reach the 1st level/grid on the bottom. 2.-put your bottles on the top of the grid cubes (covered by another "plane" grid) 3.-close it but not to tight, close the little screw where it written "fermé". 4.-make sure you put 125°C and 1,5T. 5.-it takes 35 min to sterealize (20min to reach the heating t° and 15min to heat it up till sterealized) 6.-wait till is completely cooled down 7.-open (not open till it has reached the room t°) 8.-Unplug
EXTRACTIONS:
First take some culture from the original bottle, "source". Prepare the table:
Clean it with alcohol
Fire up the gas in order to have a clean atmosphere without air bacteria
Remove the aluminium foil/protection from the mouth of the source bottle & heat it up a bit with fire the mouth of the bottle before emptying the "source"
empty the source bottle on the new bottle
heat it up a bit with fire the mouth of the bottle and the aluminium foil before putting in again the aluminium foil.
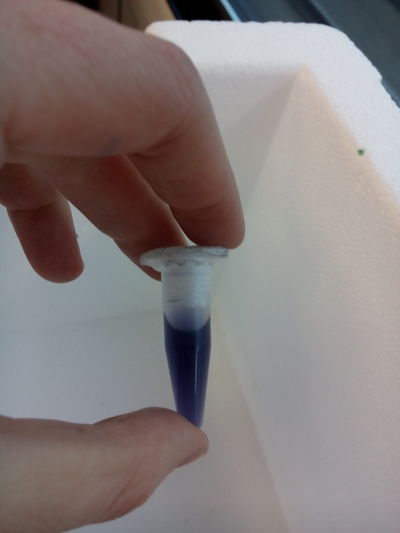
Then we continue the process with this tools: 1.- Pipette, always use the plastic cone(aeroject) on them: We extract some an put them in tubes (then close them). 2.- Then we shake the tubes in the automatic vortex at 10 x 1000 and 10 minutes 3.- Empty the lb medium that should remain on the top, we mix the rest (the pigment), with alcohol, we put the tubes in the machine of ultrasounds, in order to break the molecules to free the pigment. (ultra strong detergent would be helpful) the tube content should seem creamy and colorful. 4.- and then we put them again into the vortex in order to compact the pigment and keep it as a first sample to work with, THE FIRST YELLOW INK!!!
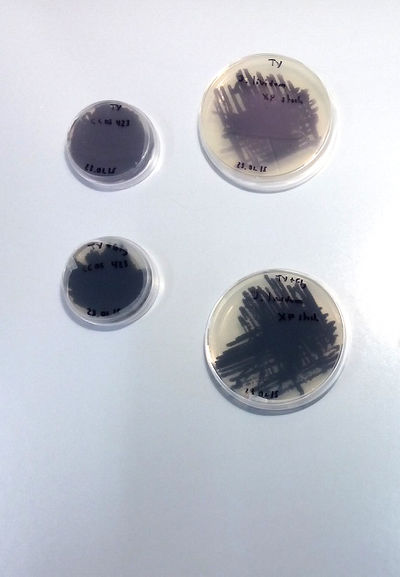
- Notes on J. Lividum***********
X.Perret, Senior Lecturer Microbiology Unit Laboratory of Microbial Genetics Sciences III – University of Geneva has accepted to collaborate in the project. We are working in the lab at UM under his supervision and the assistance of his students and Phd students. First Extraction following the article Pantanella-F_2007: [1]
Culture on TY and TY + Glycerol
SOME CONCLUSIONS: TY and TY + Gly is perfect for J.Lividum (Violet) MSA, TY not working for M.luteus (Yellow) LB + 4gr. plus of "food" (yeast and peptone) neither. MLuteus grow better on LB agar and liquid medium at 37°C (maybe not well calibrated machine...)
Extraction: Violacein extraction J. lividum was grown in 2 ml of LB or LB-U or LB-Y in 24-well plates. At regular time intervals, bacterial cells from a single well were harvested by centrifugation (16 000 g for 20 min), and treated for violacein extraction. Violacein extraction was carried out according to previously described methods (Blosser and Gray 2000; Matz et al. 2004). Briefly, bacterial cells were lysed with 10% sodium dodecyl sulfate. After 5 min at room temperature, water-saturated butanol was added (1 : 2 v/v) and vortexed. After centrifugation (16 000 g for 10 min) the upper phase, containing violacein, was collected. The extracted violacein was quantified using a spectrophotometer (OD585). The extinction coefficient of violacein (0Æ05601 ml lg)1 cm)1) was used for quantitative calculations (Mendes et al. 2001).
EXTRUSIONS--> biocoloured bioplastic:
Building the FilaBot DIY Kit, an extruder for plastics. 40 hours of intense work. Thank Michael for the moral support and electronic supervision.
Put the natural white pellets on the machine and verted the bio+ink.
The problem seems to be solved almost, if the pigments are added BEFORE putting them into the extruder let them dry (because of the extraction they already have alcohol so they dry fast) for a around 30min at room T°.
RESULT:
We could start printing with our Pr(ink)t as soon as I get to assembly and connect it!
RESULT OFFICIAL PAGE PRINT PLASTIC IS FANTASTIC
Related Links
- Test Colonies Online FREE
- hackpad for the Prinkt plastic project
- Exhibition 2015 Milano fuori salone
- Bioink project of la paillasse
- Waag Society Workshop
Resources